A Revolution in Enzyme Creation & Kit Design
As many researchers and lab technicians are aware, polymerase chain reaction, a cornerstone of biotechnology and diagnostic research, is plagued by bacterial contamination in reagents. Leading to inaccurate tests and necessitating repeat and redundant result cross checks, reagent background DNA directly impacts a researcher’s time and finances. There are many proposed, yet few, reliable solutions.
Pharmozyme has aimed to tackle this problem at the source, through a novel manufacturing method, eliminating a problem the CDC has coined “[a] contamination on a scale that’s never been reported in science before.”
In addition to strides in enzyme and mastermix design, Pharmozyme has developed an enzyme free from bacterial and animal DNA contamination. Our mission remains in making the life of researchers easier through the introduction of accurate, easy to use, and extraction free kits. We hope to be your reliable partner in PCR.
Crystal Taq™ DNA Polymerase | Crystal Taq™ PCR Master Mix
 The world’s first Taq polymerase free from bacterial and animal DNA contamination, following a breakthrough in enzyme production.
The world’s first Taq polymerase free from bacterial and animal DNA contamination, following a breakthrough in enzyme production.
- Engineered for highly sensitive applications; specifically qPCR and RT-PCR
- High-yield and zero contamination, insuring sample longevity
- Cleaner amplicons for pyrosequencing and next-generation sequencing
- This enzyme is from a recombinant source
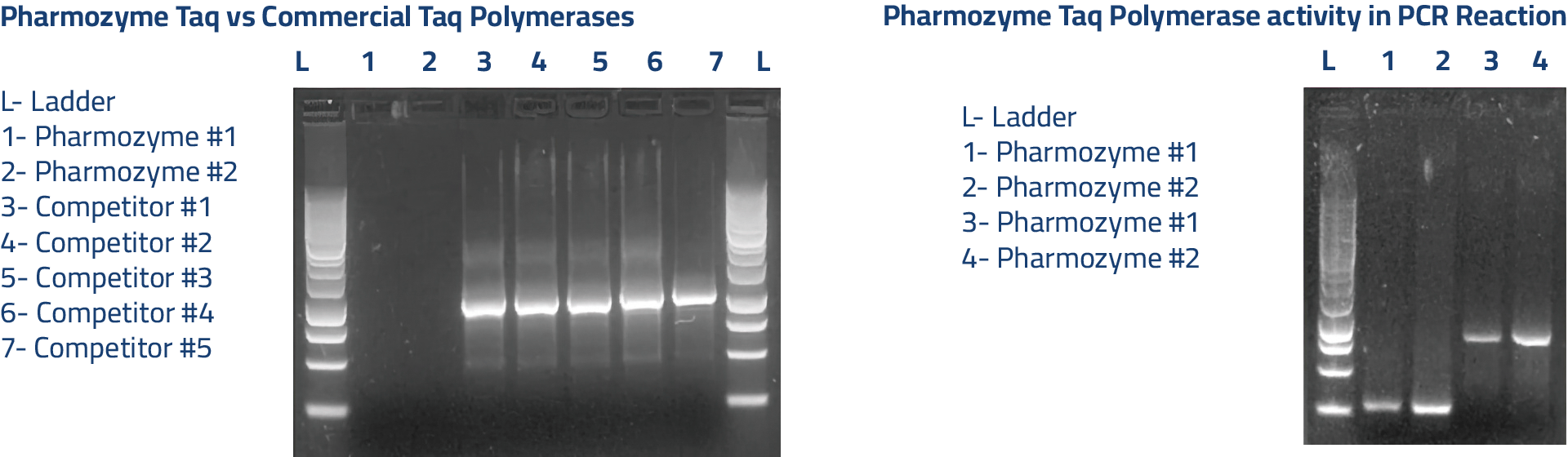
- 70 Cycle Certification – The enzyme does not show any nonspecific endo- or exo-nuclease activities. The result of 16S assay on our Crystal Taq™ DNA polymerase showed no bacterial DNA contamination after 100 PCR cycles
Samurai Taq™ DNA Polymerase
 A highly purified, low DNA Taq Polymerase, Samurai Taq™ is designed for a wide range of gene amplification applications.
A highly purified, low DNA Taq Polymerase, Samurai Taq™ is designed for a wide range of gene amplification applications.
- Low DNA background perfect for gene amplification
- Best research value – highly purified DNA Taq polymerase – minimal DNA
Ninja Taq™ RT-qPCR Master Mix

A highly purified mastermix designed for our Reverse Transcriptase applications.
“Crystal Taq™ works well in our system, and when using an assay which amplifies all bacterial DNA reduces background by about 75% (suggesting that most background comes from the Taq, but there is still a proportion from other sources).”
- Lee SmithHead of Research, Australia’s Leading Diagnostics Provider
“We performed sensitivity and clinical correlation studies on our samples to evaluate the Pharmozyme kit and happily found out that the kit performed much better than our already established and validated assays.”
- Top COVID Testing Lab Client